Abstract
Background: Increased use of oral anticancer agents (OAA) has empowered adults with chronic lymphocytic leukemia (CLL) to manage their therapy independently. However, comorbid chronic disease is common among patients with CLL, and initiating OAA therapy increases the treatment burden on adults with both cancer and multiple chronic conditions (MCC). The objective of this research was to examine adherence to OAAs in adult patients with CLL newly initiating OAA treatment and assess the extent to which such initiation impacted adherence to medications for existing comorbid chronic conditions.
Methods: This retrospective cohort study used commercial (MarketScan) and Medicare (20% sample) claims data for 2013-2018 to assess medication use in adults with CLL. To be included, patients must have been: 1) at least 18 years old; 2) diagnosed with and had 2+ claims for an OAA indicated for either CLL; 3) continuously enrolled 12 months before and after OAA initiation; and 4) treated for (2+ fills before and after OAA initiation) at least 2 select chronic conditions (diabetes, hypertension, or hyperlipidemia plus at least 1 other chronic condition). Proportion of days covered (PDC) determined medication adherence for all included medication classes and was compared for the 12 months before and after OAA initiation by Wilcoxon signed-rank tests and McNemar's tests. Multivariable logistic regression identified predictors of OAA adherence in the first year of therapy while controlling for available demographic and clinical characteristics. Difference-in-differences models assessed the change in adherence to select comorbid chronic disease medications before and after OAA initiation.
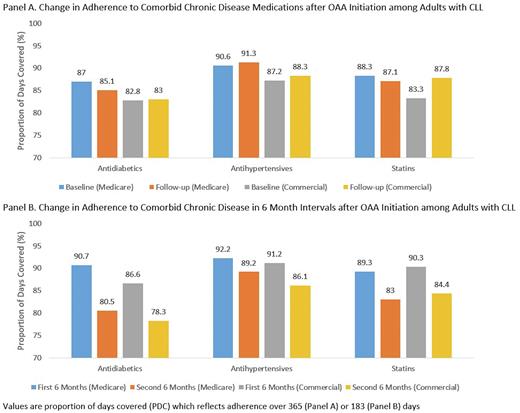
Results: The final analysis included 246 (commercial) and 805 (Medicare) adults with CLL and MCC. Mean OAA adherence in the first year of therapy was 79.8% (SD: 21.1) and 74.7% (SD: 24.9) for commercial and Medicare patients, respectively, translating to 68.3% (commercial) and 59.1% (Medicare) being adherent (PDC>80%). An increase in comorbidity burden was associated with a lower odds of OAA adherence and was the sole significant predictor in the Medicare models: OR=0.87 (95% CI: 0.82-0.92) for Medicare members and OR=0.83 (95% CI: 0.73-0.95) with commercial coverage. Among adults with commercial coverage, the odds of OAA adherence were lower for residents of the South (OR=0.39; 95% CI: 0.16-0.94; compared to the Northeast), but higher among those using a greater number of medications overall (OR=1.08; 95% CI: 1.01-1.16). No significant changes in mean adherence to MCC medications were observed, irrespective of payer, when comparing values before and after OAA initiation (Figure Panel A), including when accounting for OAA adherence in the first year of therapy. However, compared to the first 6 months post-OAA initiation, mean PDCs in the second half of the year noticeably declined, irrespective of comorbidity or payer cohort, with PDC declines ranging from 3.0 (antihypertensives, Medicare) to 11.1 (antihypertensives, Medicare) percentage points (Figure Panel B).
Conclusion: Using nationally representative claims data, this study found that initial OAA adherence was generally good among adults with CLL, with a majority of patients reaching accepted thresholds of adequate anticancer medication use. Additionally, few differences in adherence were observed in multivariable analyses using a range of patients’ characteristics, irrespective of payer or cancer type. Importantly, limited evidence of changes in adherence to oral antidiabetic, antihypertensive, or lipid-lowering agents were found in the first year following OAA initiation. However, these findings do not negate the need for careful monitoring of medication use in these populations over the course of time as some eventual declines in comorbid chronic disease medication adherence were observed.
Disclosures
Gatwood:Sanofi: Speakers Bureau; Kite: Speakers Bureau; AstraZeneca: Research Funding; GlaxoSmithKline: Research Funding; Jazz Pharmaceuticals: Speakers Bureau; Genentech: Consultancy; Janssen Pharmaceuticals: Consultancy; Merck & Co.: Consultancy, Research Funding. Dashputre:Bausch Health: Current Employment, Current equity holder in publicly-traded company; AstraZeneca: Research Funding. Rajpurohit:AstraZeneca: Research Funding. Gatwood:Kite Pharma: Speakers Bureau; sanofi: Speakers Bureau; AstraZeneca: Research Funding; Jazz Pharmaceuticals: Speakers Bureau. Mackler:mBIOHEALTH: Consultancy, Current equity holder in private company; AstraZeneca: Research Funding. Farris:AstraZeneca: Research Funding. Rizvi-Toner:AstraZeneca: Research Funding. Farley:AstraZeneca: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal